A useful carbon-carbon bond-forming reaction known as the Aldol Reaction or the Aldol Condensation is yet another example of electrophilic substitution at the alpha carbon in enols or enolate anions. Three examples of the base-catalyzed aldol reaction are shown in the following diagram, and equivalent acid-catalyzed reactions also occur. The fundamental transformation in this reaction is a dimerization of an aldehyde (or ketone) to a beta-hydroxy aldehyde (or ketone) by alpha C–H addition of one reactant molecule to the carbonyl group of a second reactant molecule.
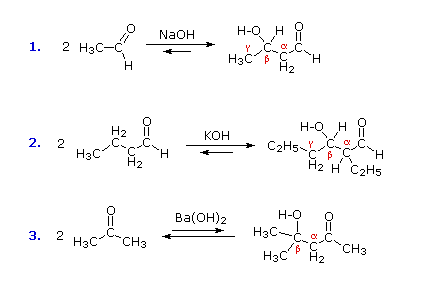
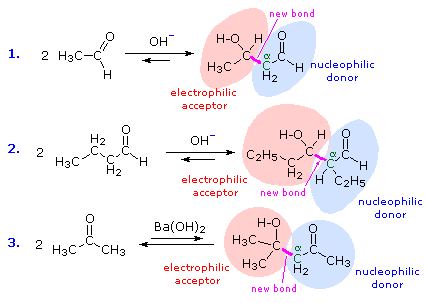
Structural Analysis
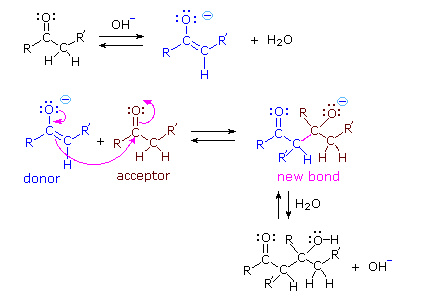
Base Catalyzed Mechanism
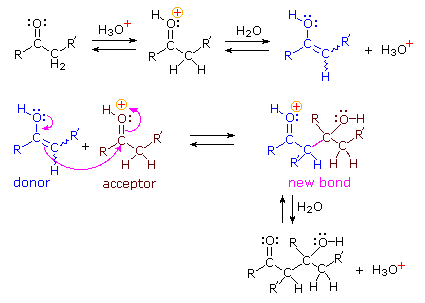
Acid Catalyzed Mechanism
Directed Aldol Reaction
Directed aldol reaction is a variation of crossed aldol reaction.
crossed aldol reaction:

If a mixture of two enolizable aldehydes, two enolizable ketones, or an enolizable aldehyde and an enolizable ketone were subjected to the conditions of crossed aldol reaction, two enolate ions would form.
eg:

Each enolate ion would react with unreacted 1 and 2, giving, after protonation by water, an aldol.

Since the overall reaction results in four aldols, it would not be an effective approach to synthesizing any of the aldols; the yield of each aldol would be poor and purification would be difficult. Aldols 3 and 6 can be prepared readily using aldol reaction. Directed aldol reaction provides a method to synthesize aldols 4 and 5 in high yield.
desired aldol = 4:

mechanism:

desired aldol = 5:

mechanism:


No comments:
Post a Comment
Note: only a member of this blog may post a comment.