Monday 24 June 2013
Wednesday 19 June 2013
Wurtz Fittig Reaction
* The Wurtz-Fittig reaction is the modification of Wurtz reaction. It involves the coupling of an aryl halide with an alkyl halide molecule in presence of sodium metal to furnish alkylated aromatic hydrocarbons.
| Dry Ether | ||
| Ar-X + 2Na + X-R | -----------------> | Ar-R + 2NaX |
Where Ar = Aryl group, R = alkyl group, X = halogen
* The more reactive alkyl halide will form the organosodium initially, which acts as a nucleophile and attacks the aryl halide.
* Usually the yields are very high.
* Refer Wurtz reaction for the reaction conditions and the detailed mechanism.
ILLUSTRATIONS
1) Toluene can be prepared by Wurtz-Fittig method as follows:
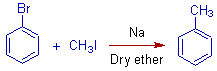
Wurtz Reaction
* In Wurtz reaction, two alkyl halide molecules are coupled in presence of sodium metal in anhydrous ether or Tetrahydrofuran to form a new carbon carbon bond and thus by giving a symmetrical alkane.
| Dry Ether | ||
| R-X + 2Na + X-R | -----------------> | R-R + 2NaX |
Where X = halogen
* The Wurtz reaction must be performed under anhydrous conditions because the alkyl free radical formed (see the mechanism) during the reaction is strongly basic and can abstract proton from water.
* In case of alkyl and aryl fluorides as well as aryl chlorides, tetrahydrofuran is used as solvent instead of ether.
* The Wurtz reaction is limited to synthesis of symmetrical alkanes with even number of carbon atoms only. The number of carbons in the alkane is double that of alkyl halide (n ---> 2n type reaction)
* If dissimilar alky halides are used, a mixture of alkanes is formed. It is usually difficult to separate the mixture and hence wurtz reaction not a suitable method to synthesize unsymmetrical alkanes.
E.g. The Wurtz reaction between R-X and R'-X yields not only R-R' but also R-R and R'-R'. This mixture cannot be separated easily.
* Methane cannot be prepared by this method.
* A modification of this reaction involving alkyl and aryl halides is called Wurtz-Fittig reaction. If only aryl halides are subjected to coupling, the reaction is called as Fittig reaction.
MECHANISM OF WURTZ REACTION
* Initially an alkyl free radical is formed due to transfer of one electron from sodium atom.
| R-X | + | Na | ---------> | R. | + | X- |
| Alkyl free radical |
* In the next step, one more electron is transferred from second sodium atom to the free radical to give a carbonium ion.
| R. | + | Na | ---------> | R-Na+ | ||
* Thus formed alkyl anion displaces halide ion from the second molecule of alkyl halide. It is an SN2 reaction.
| R- Na+ | + | R-X | ---------> | R-R | + | Na+X- | |
| Symmetrical Alkane |
Comment: Since the alkyl free radicals are formed, elimination side reactions leading to alkenes is also possible, especially with bulky alkyl groups, which require more activation energy during the nucleophilic substitution (SN2) step.
ILLUSTRATIONS
1) Ethane is formed when methyl chloride is treated with sodium metal in dry ether.
| Dry Ether | ||
| CH3-Cl + 2Na + Cl-CH3 | -----------------> | CH3-CH3 + 2NaCl |
2) Strained carbon skeletons like bicyclobutane ( bicyclo[1.1.0]butane ) can be prepared by an intramolecular Wurtz reaction as shown below.
![preparation of bicyclo[1.1.0]butane by wurtz reaction](http://www.adichemistry.com/organic/namedreactions/wurtz/wurtz1-1.gif)
3) When tert-butylhalides are subjected to Wurtz reaction, isobutylene is formed as the major product. It is because the elimination is favored over SN2 mechanism. The SN2 step requires more activation energy due to steric hindrance.
Reformatsky Reaction
* The Reformatsky reaction involves the treatment of an α-halo ester with zinc metal and subsequent reaction with aldehyde/ketone to get β- hydroxy ester.
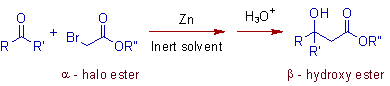
* Usually inert solvents like diethyl ether or THF are used in Reformatsky reaction.
* Better yields are obtained by using Zn-Cu couple or in situ preparation of zinc by reduction of zinc halides by potassium (also known as Rieke zinc).
Mechanism
* Initially zinc reacts with α-halo ester to give an organozinc reagent called reformatsky enolate. It is just like the Grignard reagent. It is added to the carbonyl group of aldehyde or ketone to furnish β- hydroxy ester.
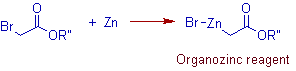
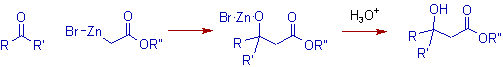
* The organozinc reagents are less reactive and hence the nucleophilic addition to the ester group seldom occurs. Some of them are quite enough stable to be isolated and can be elucidated for the structure by techniques like X-ray analysis.
ILLUSTRATIONS
1) The following reaction is a classical example of Reformatsky reaction.
| Zn | H3O+ | |||
| CH3CHO + Br-CH2-COOC2H5 | -----------------> | -----------> | CH3CH(OH)-CH2COOC2H5 | |
| Diethyl ether |
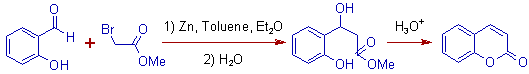
2) The Reformatsky reaction is involved in the formation of following β- hydroxy ester, which upon condensation gives Coumarin as the final product.
Hunsdiecker Reaction
The decarboxylation of silver salts of carboxylic acids to alkyl bromides by treating with bromine is known as Hunsdiecker reaction. The alkyl bromide contains one carbon less than those in carboxylic acid.
This reaction is also known as Borodin-Hunsdiecker reaction.
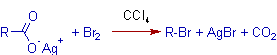
* Very good yields are obtained with alkyl groups containing 2 to 18 carbons. This reaction works with linear as well as branched chains. However the reaction seldom works with alkyl groups containing unsaturation.
* This reaction is usually carried out in carbon tetrachloride solvent.
* Although bromine is used often, the reaction is also possible with chlorine and iodine.
* When iodine is used, the ratio between the silver carboxylate and iodine is very important and determines the products.
A 1:1 ratio of silver salt and iodine gives the alkyl halide.
However, an ester, RCOOR is formed when the reaction is carried out with a 2:1 ratio of silver carboxylate and iodine. This is called as Simonini reaction.
* Incase of aromatic carboxylates, the Hunsdiecker reaction is possible when the aromatic ring contains electron-withdrawing groups.
Otherwise, if the aromatic system contains electron-donating groups, the bromine will substitute one of the hydrogen on the aromatic ring rather than promoting the Hunsdiecker reaction.
However the use of NBS instead of bromine will give the desired Hunsdiecker product. This reagent is especially useful since it produces bromine free radicals slowly.
* The silver carboxylate used as the starting material must be sufficiently pure and dry. It can be prepared from the corresponding carboxylic acid by treating it with silver oxide, Ag2O.
Christol-Firth Modification: It is possible to perform the Hunsdiecker reaction conveniently on the free carboxylic acid instead of the silver salt, which otherwise requires purification. In this modification the free carboxylic acid is treated with a mixture of mercuric oxide, HgO and bromine in CCl4. There is no need to isolate an intermediate salt.
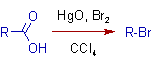
Mechanism
Initiation: Initially the bromine reacts with the silver carboxylate to give an unstable acyl hypobromite. The driving force of this step is the precipitation of the extremely poorly soluble and stable AgBr.
The acyl hypobromite decomposes by homolytic cleavage of relatively weak O-Br bond to furnish an acyl free radical.
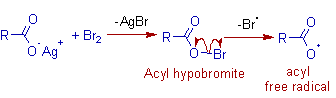
Propagation: The acyl free radical undergoes decarboxylation to furnish an alkyl free radical, which reacts with acyl hypobromite to give the final product alkyl bromide along with the formation of a new acyl free radical.
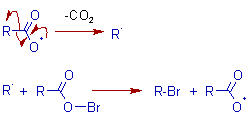
The following facts support the above proposed free radical mechanism for Hunsdiecker reaction.
i) No rearrangement of alkyl groups
ii) The formation of side products like R-R.
iii) If the alkyl group, R is chiral, it loses its optical activity during this reaction.
ILLUSTRATIONS
1) The silver salt of propionic acid is converted to ethyl bromide when treated with bromine in tetrachloromethane.
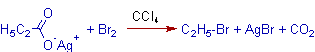
2) In the following reaction, the use of NBS (N-Bromosuccinimide) reduces the chances of electrophilic substitution on benzene ring.
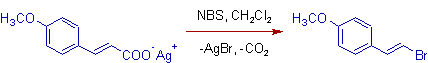
3) The Christol-Firth Modification is used in the preparation of [1.1.1]propellane (tricyclo[1.1.1.01,3]pentane). The conversion of Bicyclo[1.1.1]pentane-1,3-dicarboxylic acid to the corresponding dibromide is achieved by using mercuric oxide and bromine in carbon tetrachloride as shown below.
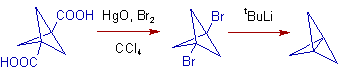
Corey-House Synthesis
The Corey–House synthesis (also called the Corey–Posner, Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium dialkyl cupratewith an alkyl halide to form a new alkane, an organocopper compound and a lithium halide
R2CuLi + R'-X → R-R' + RCu + LiX
R2CuLi + R'-X → R-R' + RCu + LiX
This reaction occurs in two steps. The alkyl halide is treated with lithium metal, and solvated in ether, which converts the alkyl halide into an alkyl lithium compound, R-Li. The starting R-X can be primary, secondary or tertiary alkyl halide:
- R-X + 2Li → R-Li + Li-X
The second step requires the alkyl lithium compound to be treated with cuprous iodide (CuI). This creates a lithium dialkyl cuprate compound. These compounds were first synthesized by Henry Gilman of Iowa State University, and are usually called Gilman reagents in honor of his contributions:
- 2RLi + CuI → R2CuLi + LiI
The lithium dialkyl cuprate is then treated with the second alkyl halide, which couples to the compound:
- R2CuLi + R'-X → R-R' + RCu + LiX
If second alkyl halide is not the same as the first, then cross-products are formed.
It is important to note that for this reaction to work successfully, the second alkyl halide must be a methyl halide, benzyl halide, primary alkyl halide or a secondary cyclo alkyl halide. The relative simplicity of this reaction makes it a useful technique for synthesizing organic compounds.
Mechanisms in Organic Chemistry
Important Mechanisms from Organic chemistry for +2 level Exams....
1. Free Radical substitution in Alkane (Halogenation)
2. Electrophilic Aubstitiutionddition to Alkenes and Alkynes
3. Electrophilic Aromatic Substitution (EAS)
4. Nucleophilic Substitution at saturated carbon atom (SN1, SN2, SNi, NGP)
5. Eliminations (E1, E2, E1cb)
6. Nucleophilic substitution in Activated Aromatic compounds (ArSN)
7. Nucleophilic substitution in Non-activated Aromatic compounds (Benzyne)
8. Nucleophilic Additions to Carbonyl group (aldehyde and ketones)
9. Nulcleophilic Substitution at Carbonyl carbon atom.
1. Free Radical substitution in Alkane (Halogenation)
2. Electrophilic Aubstitiutionddition to Alkenes and Alkynes
3. Electrophilic Aromatic Substitution (EAS)
4. Nucleophilic Substitution at saturated carbon atom (SN1, SN2, SNi, NGP)
5. Eliminations (E1, E2, E1cb)
6. Nucleophilic substitution in Activated Aromatic compounds (ArSN)
7. Nucleophilic substitution in Non-activated Aromatic compounds (Benzyne)
8. Nucleophilic Additions to Carbonyl group (aldehyde and ketones)
9. Nulcleophilic Substitution at Carbonyl carbon atom.
Saturday 15 June 2013
Crossed Claisen Condensation
Crossed Claisen condensation is a variation of Claisen condensation.
Claisen Condensation:

Crossed Claisen Condensation:

eg:

mechanism:
Step 1: The alkoxide ion deprotanates the enolizable ester reversibly.
Step 1: The alkoxide ion deprotanates the enolizable ester reversibly.

Step 2 and 3: Enolate ion 1 undergoes a nucleophilic acyl substitution preferentially with the non-enolizable ester, which has the sterically less hindered and, therefore, more accessible carbonyl carbon, giving a β-ketoester.

Step 4: The alkoxide ion deprotonates the β-ketoester irreversibly.

Step 5: The acid protonates enolate ion 2

Wittig Reaction
The Wittig reaction is the reaction of a Wittig reagent with an aldehyde or a ketone to afford an alkene as the organic product.
eg:

Mechanism:

Williamson Ether Synthesis
Williamson ether synthesis is a method of preparation of ethers. It is a nucleophilic aliphatic substitution at saturated carbon in which thenucleophile is either an alkoxide ion or a phenoxide ion.

R2 = alkyl, aryl
eg:
Some symmetrical ethers can not be prepared using Williamson either synthesis because, in addition to nucleophilic aliphatic substitution,1,2-elimination could occur between a substrate bearing beta hydrogens and an alkoxide ion, leading to an alkene as the organic product.
eg:

To prepare ether 1 using Williamson ether synthesis, a tert-butyl substrate (2) should be reacted with the tert-butoxide ion (3).

Since alkoxide ions are strongly basic and since the substrate is a tertiary alkyl substrate, the major reaction between 2 and 3 would be 1,2-elimination, giving alkene 4 as the organic product.

Preparation of unsymmetrical ethers using Williamson ether synthesis requires planning because, again, in addition to nucleophilic aliphatic substitution, 1,2-elimination could occur between some substrates and alkoxide ion and could be the dominant process.
eg:

Wagner-Meerwein Rearrangement
A Wagner-Meerwein rearrangement is any reaction in which the carbon skeleton of a reactant changes due to one or more rearrangementsinvolving carbocations.
eg:

mechanism:

Sandmeyer Reaction
Each of the following three reactions of aromatic diazonium ions is called a Sandmeyer reaction.

Each reaction, overall, is a nucleophilic substitution.

None of the reactions, however, occurs in the absence of copper (I) ion, which is a reducing agent. The mechanism of Sandmeyer reactions is not fully understood.
Ruff Degradation
The Ruff degradation is a synthetic protocol used to remove one carbon atom from the molecule of an aldose.

eg:

Michael Addition
1,4-Addition reactions of α, β–unsaturated carbonyl compounds and α, β–unsaturated nitriles with resonance-stabilized carbon nucleophiles, such as enolate ions and enamines, are known as Michael addition. The α, β–unsaturated compounds undergoing Michael addition is called the Michael acceptor, the nucleophile Michael donor, and the product Michael adduct.
eg:

mechanism:


Mannich Reaction
The acid-catalyzed reaction of an enolizable aldehyde or an enolizable ketone with an imminium ion, usually generated in situ by the reaction of formaldehyde with a secondary amine, followed by a base to give a β-aminoaldehyde of a β-aminoketone, respectively, is known as the Mannich reaction. The product of the Mannich reaction is called the Mannich base.
eg:

mechanism:

Malonic Ester Synthesis
Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2.

R1 = alkyl group
L = leaving group
The group —CH2CO2H in 2 is contributed by a malonic ester, hence the term malonic ester synthesis.

R2 = alkyl, aryl
Malonic ester synthesis consists of four consecutive reactions that can be carried out in the same pot.
reaction 1: acid-base reaction
reaction 2: nucleophilic substitution
reaction 3: ester hydrolysis (using saponification)
reaction 4: decarboxylation
reaction 2: nucleophilic substitution
reaction 3: ester hydrolysis (using saponification)
reaction 4: decarboxylation
eg:

reaction 1:

reaction 2:

reaction 3:

reaction 4:

A more direct method to convert 3 into 4 is the reaction of 3 with the enolate ion (5) of ethyl acetate followed by hydrolysis of the resultantester.

However, the generation of 5 from ethyl acetate quantitatively in high yield is not an easy task because the reaction requires a very strongbase, such as LDA, and must be carried out at very low temperature under strictly anhydrous conditions.

Malonic ester synthesis provides a more convenient alternative to convert 3 to 4.
Malonic ester synthesis can be adapted to synthesize compounds that have the general structural formula 6.

R3, R4 = identical or different alkyl groups
eg:

reaction 1:

reaction 2:

reaction 1 (repeat):

reaction 2 (repeat):

reaction 3:

reaction 4:

Subscribe to:
Posts (Atom)

